- The nucleus is represented by the atomic symbol
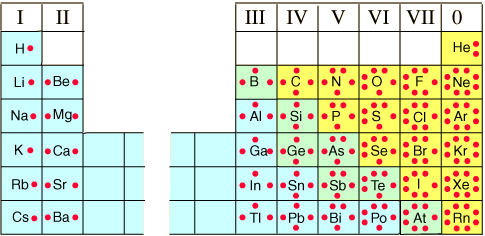
- For individual elements determine the number of valence electrons
- Electrons are represented by dots around the symbol
- Four obitals (one of each side of the nucleus each holding a max of 2e-)
- Each orbital get 1e- beofre they pair.
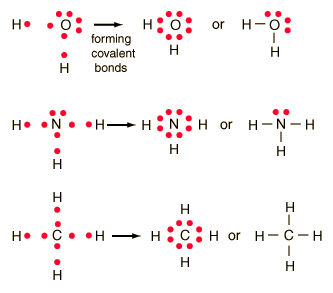
A) Lewis Diagrams for compounds and ions
In covalent compounds electrons are shared.
- Determine the number of valence electrons for each atom in the molecule
- Place the atoms so that the valence electrons are shared to fill each orbital
B) Double and Triple Bonds
Sometimes the only way covalent compounds can fill all their valence levels is if they share more than one electron.
C) Ionic Compounds
- In Ionic compounds electrons transfer from one element to another
- determine the number of valence electrons on the cation. move these to the anion
- Draw [ ] after the metal and non metal.
- Write the charges outside the brackets






No comments:
Post a Comment