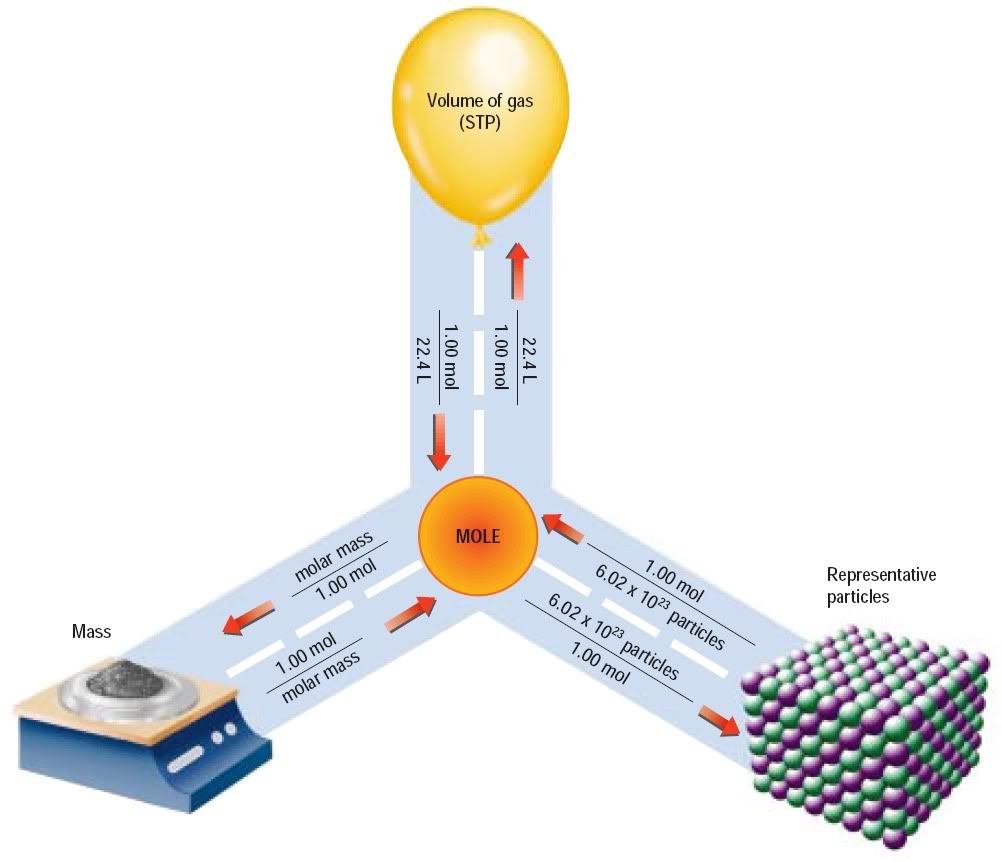
- At a specific pressure and temperature 1 mole of any gas occupies the same volume,
- At 0 °C and 101.3 kPa, 1mol = 22.4L
- This temperature and pressure is called STP
- STP = Standard Temperature and Pressure
- 22.4L/ mol is the molar volume at STP
Examples:
How many litres will 2.5 mol of H2 occupy at STP?
2.5 mol x 22.4 L/ 1 mol= 56 L
A certain gas is found to occupy 11.6L at STP. How many moles of gas are there?
11.6L x1 mol/ 22.4 L= 0.518 mol.
At STP a sample of oxygen gas contains 11.5 mol. How many L of oxygen are there?
11.5 mol x 22.4 L/ 1 mol = 257.6 L= 258L
At STP, an unknown gas is found to occupy 150 mL. How many moles of Gas must there be?
There are two methods of solving this equation:
1.
150mL x 1L/1000mL= 0.150L
0.150L x 1 mol/ 22.4L = 0.0670 mol or 6.70 x 10^-3
Or uses
2.
(150mL) x (1L/ 1000mL) x (1mol/22.4 L)= 0.00670mol
A certain amount of chlorine gases occupies 1.6L. Find the number of moles present and then determine the mass of chlorine.
1.6L x 1mol/22.4L= 0.071 mol
0.071 mol x 71 g/ 1mol= 5.1g
(1.6L) x (1 mol/ 22.4 L)x (71g/ 1mol)= 5.1 g
No comments:
Post a Comment