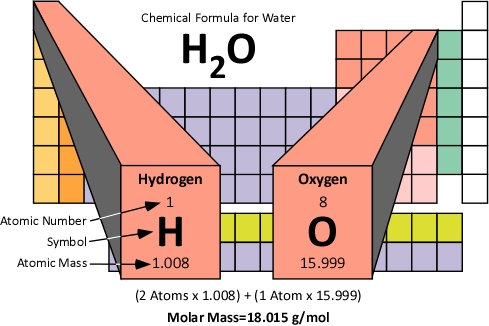
- The mass in grams of 1 mole of a substance is called a “Molar Mass”
- It can be determined from the atomic mass on the periodic table.
- Measured in g/mol.
- Some common masses are:
- Hydrogen =1.0079
- Helium = 4.0
- Nitrogen = 14.01
- Zinc = 65.37
- Iron = 55.845
- Sodium = 22.9
- Uranium = 238.03
1) Molar Mass
- The atomic mass----> Molar Mass
- The molar mass is the weight of one mole or 6.02 x 1023 molecules.
- The base SI Unit for mass is the Kilogram.
- To determine the molar mass of a compound, add the mass of all the atom together.
- Always have 2 or more significant digits.
- For example: Find the Molar Mass of the compound CO2 (Carbon Dioxide)
- Atomic mass carbon = 12.01
- Atomic mass of oxygen = 16.00
- Molecular Mass for Carbon monoxide = the atomic mass carbon + the atomic mass oxygen
- 12.01 + 2(16.00) = 44.01 g/mole
Mass<-(Molar Mass)->Moles<-(Molar Volume)->Volume
- Volume at STP (Standard Temperature and Pressure)
- The Volume is always the same as long as it is the same STP.
- The Volume only applies to gases.
- 1 mole of any gas occupies the same volume.
- At 0 degrees celsius and 101.3 kPa (1 mol=22.4L)
- 22.4L/mol is the molar volume at STP
- KEY information to remember:
1 mol = g-formula-mass (periodic table)
1 mol = 22.4 L for a gas at STP
Examples:
1. A certain gas is found to occupy 0.040 moles at STP. Find the volume in liters.
(0.040 mol) x(22.4 L/ 1 mol) = 0.896L
2. How many moles of Sulfur atoms are present in 11.2 L of Sulfur gas at STP?
(11.2L)x(1mol/ 22.4L) = 0.5 mol
No comments:
Post a Comment